There is a good diary currently in the rec list today about global warming (Intro to Basic Science of Global Warming). I noticed among the comments was a request for a primer in paleoclimate work. When the poster said that he would leave such posts for more experienced people, I realized I wouldn't be stepping on any toes by stepping in. As it turns out, I posted just such a primer on my facebook page solely for my family a few months back, but I see no reason that I can't share it with the community at large. I have a number of these that I put together for my family so they could understand what I do. If there is interest maybe I will get around to posting the rest.
I define myself as a paleoceanographer (study of the past ocean), but the terms micropaleontologist (study of micro-fossils), paleoclimatologist (study of past climate), marine geologist/geological oceanographer (interchangeable, both are the study of the geology of the ocean), and most importantly it would seem geochemist (study of the cycling of various elements on our planet) are all relevant. My interests fundamentally lie in changes in the ocean's circulation patterns with time and I have approached this scientific curiosity from several different angles. I will start with the most basic paleoclimatic information, and expand from there if people are interested. So today, foraminifera and oxygen isotopes!

 When we first bring up sediments from the seafloor, they contain a mixture of silicates (material eroded from the continents and carried to the ocean via riverine or wind transport) and biogenics (fossils of dead micro-organisms). The first picture above is from sediments along the California margin that are 80-90% silicates, while the second picture of sediments from the Equatorial Pacific is 100% biogenic in nature. Depending on where you take the sediments from, the ratio of these two components can very drastically (there are other components too, such as volcaniclastic sediments which are made up of ash and glass, hydrogenous sediments which are made up of sediments that actually form in the ocean, and cosmogenic sediments which are made up of material derived in space but they all form a minor component of seafloor sedimentation except in unusual circumstances so I will ignore them for now). I am largely interested in the biogenic portion of the sediments. There are a number of different groups that leave behind an amazing fossil record, some you may remember hearing about in high school biology. The predominant sedimentary fossils include the foraminifera, the calcareous nannoplankton (coccolithophorids and discoasters), diatoms, and radiolarians.
When we first bring up sediments from the seafloor, they contain a mixture of silicates (material eroded from the continents and carried to the ocean via riverine or wind transport) and biogenics (fossils of dead micro-organisms). The first picture above is from sediments along the California margin that are 80-90% silicates, while the second picture of sediments from the Equatorial Pacific is 100% biogenic in nature. Depending on where you take the sediments from, the ratio of these two components can very drastically (there are other components too, such as volcaniclastic sediments which are made up of ash and glass, hydrogenous sediments which are made up of sediments that actually form in the ocean, and cosmogenic sediments which are made up of material derived in space but they all form a minor component of seafloor sedimentation except in unusual circumstances so I will ignore them for now). I am largely interested in the biogenic portion of the sediments. There are a number of different groups that leave behind an amazing fossil record, some you may remember hearing about in high school biology. The predominant sedimentary fossils include the foraminifera, the calcareous nannoplankton (coccolithophorids and discoasters), diatoms, and radiolarians.
 I am predominantly interested in the foraminifera or forams for short (see photos). Foraminifera are single-celled protists that are from the Ameoboid group called the Sarcodinians. They are mobile, but they move by extending pseudopodia of cytoplasm and dragging themselves after it rather than the more complex cilia or flagella used by other protozoans. They eat anything that they can engulf via phagocytosis (the process of engulfing your food in cytoplasm and bringing it into your cell for digestion). Mostly they feed on bacteria and diatoms, but some of the larger species have been observed to eat multi-cellular organisms such as copepods or other small zooplankton. They can be as small as 10-20 microns in diameter, to several centimeters. In fact, the largest unicellular organism alive today is a foram. The limestone the great pyramids in Egypt are made of are lithified forams, and one predominant species was so large that early European archeologists erroneously labeled them as fossil lentils fed to the slaves who built the pyramids. They are also the only eukaryotic group to include a species that has been demonstrated to live off of an alternative electron acceptor (nitrogen instead of oxygen, which is amazing, but only if you are a biologist). Aside from all of that, and a billion other neat things that I could expound on forever, what sets them in the hearts of geological oceanographers like myself is that they form an exoskeletal shell, called a "test" of calcium carbonate (CaCO3) or calcite for short. This is important because while the cell itself cannot
I am predominantly interested in the foraminifera or forams for short (see photos). Foraminifera are single-celled protists that are from the Ameoboid group called the Sarcodinians. They are mobile, but they move by extending pseudopodia of cytoplasm and dragging themselves after it rather than the more complex cilia or flagella used by other protozoans. They eat anything that they can engulf via phagocytosis (the process of engulfing your food in cytoplasm and bringing it into your cell for digestion). Mostly they feed on bacteria and diatoms, but some of the larger species have been observed to eat multi-cellular organisms such as copepods or other small zooplankton. They can be as small as 10-20 microns in diameter, to several centimeters. In fact, the largest unicellular organism alive today is a foram. The limestone the great pyramids in Egypt are made of are lithified forams, and one predominant species was so large that early European archeologists erroneously labeled them as fossil lentils fed to the slaves who built the pyramids. They are also the only eukaryotic group to include a species that has been demonstrated to live off of an alternative electron acceptor (nitrogen instead of oxygen, which is amazing, but only if you are a biologist). Aside from all of that, and a billion other neat things that I could expound on forever, what sets them in the hearts of geological oceanographers like myself is that they form an exoskeletal shell, called a "test" of calcium carbonate (CaCO3) or calcite for short. This is important because while the cell itself cannot  fossilize, the test can. They come in an unimaginable variety of shapes and sizes, some beautiful and delicate (like the appropriately named H. elegans), some tough and stout (like the equally appropriately named N. pachyderma). This fossil record is amazing, and makes the terrestrial fossil record (dinosaurs, mammoths, and what-have-you) look pathetic. You can study patterns of evolution, and Darwin's theories are proven true every day. There are no missing links, or gaps in the record. Both gradualism and punctuated equilibrium operate simultaneously, often times on a single species. Major extinctions (here's looking at you K/T boundary) are recorded well, while other events that severely impacted the land animals didn't touch the forams. Benthic (bottom dwelling) foraminifera first evolved from shell-less amoeboids in the Cambrian over 500 million years ago, and the planktonic (living in the upper 200 meters of the water column) first appear approximately 225 million years ago, during the Triassic. I will focus the rest of this note on the planktonic forams, as they live in the surface ocean, and were the first tests of paleoclimatic research.
fossilize, the test can. They come in an unimaginable variety of shapes and sizes, some beautiful and delicate (like the appropriately named H. elegans), some tough and stout (like the equally appropriately named N. pachyderma). This fossil record is amazing, and makes the terrestrial fossil record (dinosaurs, mammoths, and what-have-you) look pathetic. You can study patterns of evolution, and Darwin's theories are proven true every day. There are no missing links, or gaps in the record. Both gradualism and punctuated equilibrium operate simultaneously, often times on a single species. Major extinctions (here's looking at you K/T boundary) are recorded well, while other events that severely impacted the land animals didn't touch the forams. Benthic (bottom dwelling) foraminifera first evolved from shell-less amoeboids in the Cambrian over 500 million years ago, and the planktonic (living in the upper 200 meters of the water column) first appear approximately 225 million years ago, during the Triassic. I will focus the rest of this note on the planktonic forams, as they live in the surface ocean, and were the first tests of paleoclimatic research.
The fact that these shells are made of CaCO3 is of primary importance, but to explain why, I have to go back to high school chemistry. Forgive me if you think I am underestimating your intelligence gentle reader, but having taught several hundred college students, I have learned not to take any for granted. All matter in the universe is made up of atoms. Atoms are made up of subatomic particles called protons (positively charged), neutrons (neutrally charged), and electrons (negatively charged). An atom is defined by the number of protons it has, so calcium (Ca) always has 20 protons and oxygen (O) always has 8 protons. An ion is an atom with a charge on it. A stable oxygen atom would have 8 protons and 8 electrons, but an oxygen ion generally has 8 protons and 10 electrons, giving two extra negative charges (O2-). Isotopes are variations of atoms with different numbers of neutrons in their nucleus. 99.757% of oxygen atoms have 8 protons and 8 neutrons for an atomic mass of 16, but there are two other stable isotopes of oxygen, oxygen-17 (17O - 0.038%) with 8 protons and 9 neutrons, and oxygen-18 (18O - 0.205%) with 8 protons and 10 neutrons. This is very important for our understanding of paleoclimate, but first I have to explain the non-biological cycling of oxygen on our planet.
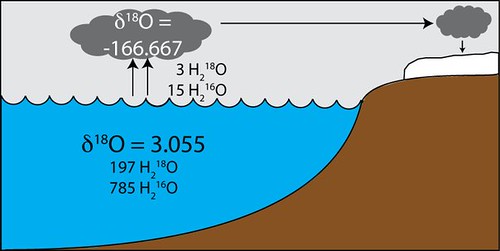
The vast majority of oxygen available for biological processes is tied up in water (H2O), the bulk of which is in the ocean (obviously). As I mentioned before there are multiple isotopes of oxygen, so water can be made up of two hydrogen atoms and an oxygen-16 (H216O, weight 18 atomic mass units (amu)), two hydrogens and an 17O (H217O, weight 19 amu), or two hydrogens and an 18O (H218O, weight 20 amu). Now, H218O is two amu heavier than H216O, which means it takes more energy for it to evaporate than the lighter form of water. Think of it as it takes more energy to lift a 200 pound person vs. an 180 pound person. The proportional weight difference is the same, just on a much much much smaller scale. So what does this mean? Well, when water evaporates out of the ocean, it evaporates more H216O relative to H218O, leaving more H218O in the ocean as a total proportion than there was before. If the water then rains out and flows back to the ocean you are left with a zero-sum game, but when you sequester that water on land, say in ice sheets, than you leave behind an ocean that is enriched in H218O relative to what it was before. Geochemists say that the ocean is "heavier" in this scenario. If you had a way to determine the 18O/16O ratio of the ocean, you could determine the extent of ice growth on the continents. This 18O to 16O ratio is difficult to work with, so, for ease of use, geochemists compare the raw ratio to that of a standard and report this result as "delta notation" δ18O. A higher number means a higher 18O/16O ratio, and therefore more ice sequestration. The highest numbers during the last glacial would be around δ18O = 5. Below is an example of how evaporation fractionates oxygen isotopes and the resulting values.
Recall that I said that the shell of a foraminiferan is made of CaCO3. That means that if you dissolve the shell, for every one atom of Ca that you release, you will release 3 oxygen atoms. You can use the oxygen isotope ratios within the foraminiferan test to determine the δ18O ratio of the ocean. But as it turns out there is an additional component of this process when you are dealing with foraminifera. Forams do not incorporate the oxygen into their shells in the 18O/16O ratio of the water around them. But rather than a minus it is a big big plus. Turns out that there is a strong temperature component in the rate at which forams incorporate 18O into their shells. To drastically over-simplify a complex thermodynamic problem, think of the atoms in the water as moving around and they are all given the same amount of energy to move. The 18O atoms will be moving slower than the 16O because it takes more energy to move them. At cold temperatures, as the foram is building its CaCO3 shell, and is trying to "grab" oxygen atoms (actually in the form of CO32- ions in seawater, but that is a topic for a whole other post), the heavier 18O atoms are easier to "catch" and incorporate into their shells than the faster 16O. This has the effect of concentrating the 18O atoms within the shell, and increasing the CaCO3 δ18O at cold temperatures. But as the temperature in the water increases, all of the atoms begin to move faster. The heavier 18O atoms begin to increase their speed faster than the 16O atoms so that, even though they are still moving slower, the difficulty of catching 16O atoms compared to 18O atoms decreases, bring the δ18O of the shell closer to the ratio of the seawater that the shell was grown in. If you know what the δ18O of the seawater that the shell was grown in, you can calculate the temperature of the water. This thermodynamic ratio, hypothesized by Harold Urey in 1947, demonstrated by Samuel Epstein in 1953 in the lab and used as a paleothermometer for the first time by Cesare Emiliani in 1954, allows paleoclimatologists such as myself to create temperature records for any given location in the past. If you generate enough of these records in enough locations you can begin to model how the whole planet's heat balance has evolved over time. This is one way that we know that the current warming on the planet is unprecedented in the history of our species.
This is the first step toward understanding how our planet works, but as you probably noticed, I did not mention anything about ocean circulation changes. That is because you need more data than temperature before you can start talking about circulation changes, but this is where you start. Obviously, much of this was oversimplified, as there are entire books written on the subject, but to the first order it is all correct. And it is certainly better than Wikipedia's entry on δ18O. Future posts will revolve around other geochemical analyses, such as nitrogen, carbon, and magnesium, as well as some of the more novel techniques like neodymium isotopes, which is what I work with.
I leave you with a photo of the United States' only scientific drilling vessel, the JOIDES Resolution that I took during a port call in Honolulu. Without the invaluable services that this vessel provides, our understanding of climate change would be immeasurably diminished. The program that funds scientific ocean drilling is currently up for reevaluation, as it expires in 2012, and the government is undecided as to whether or not to continue funding the project. I encourage you to go to the ship's website JOIDES Resolution to learn more about the good work done by our scientists at sea.
